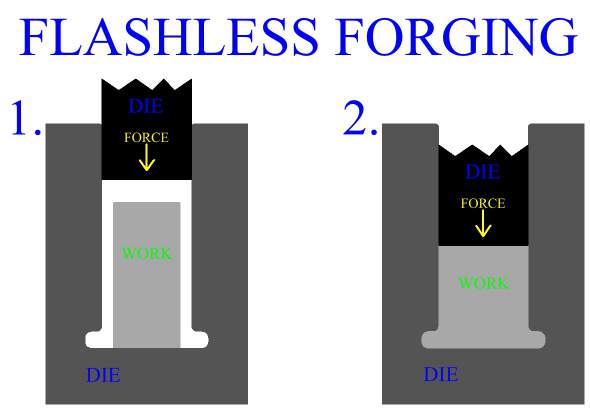
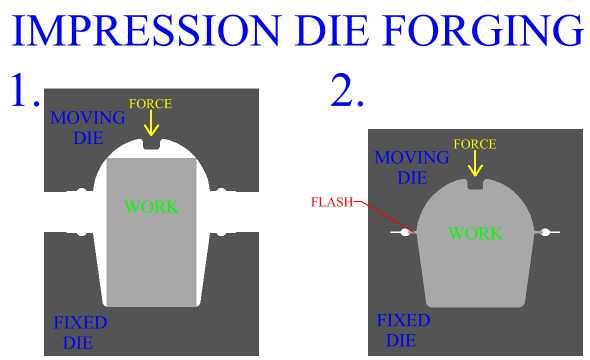
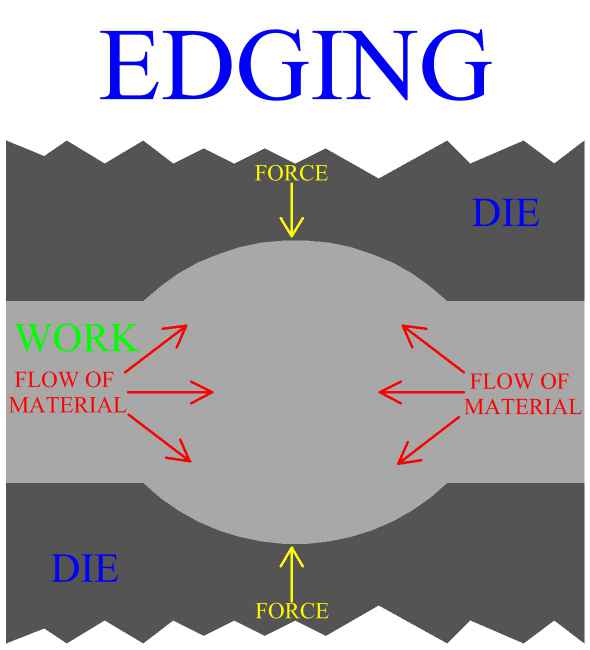
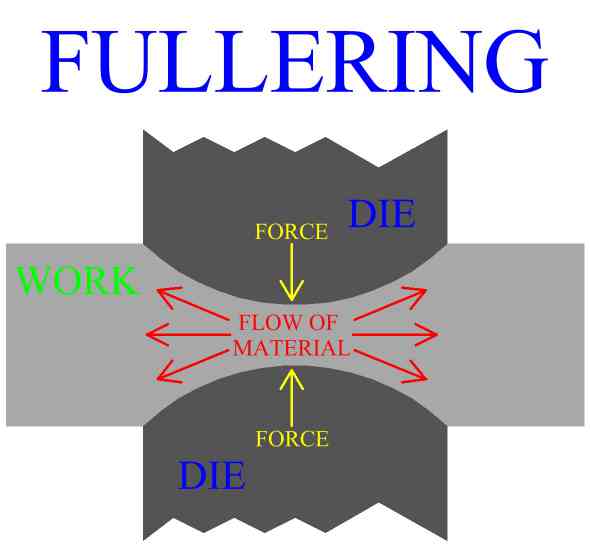
Inspection is an important aspect of metal forging manufacture. All parts should be checked for defects after the manufacturing process is complete. Defects of metal forged product include exterior cracking, interior cracking, laps, cold shuts, warping of the part, improperly formed sections and dead zones.
In general, defects in parts manufactured by metal forging can be controlled first by careful consideration of work stock volume, and by good design of both the forging die, (mold), and the process. The main principle is to enact the right material distributions, and the right material flow to accomplish these distributions. Die cavity geometry and corner radius play a large roll in the action of the metal. Forging die design, and forging process design will be discussed in later sections.
Go back to Forging
Cracking
Cracking both interior and exterior is caused by excessive stress, or improper stress distribution as the part is being formed. Cracking of a forging can be the result of poorly designed forging die or excess material in the work piece. Cracks can also be caused by disproportionate temperature distributions during the manufacturing operation. High thermal gradients can cause cracks in a forged part.Laps or folds
Laps or folds in a metal forging are caused by a buckling of the part, laps can be a result of too little material in the work piece.Cold shuts
Cold shuts occur when metal flows of different temperatures meet, they do not combine smoothly, a boundary layer, (cold shut), forms at their intersection. Cold shuts indicate that there is a problem with metal flow in the mold as the part is being formed.Warping
Warping of a forged part can happen when thinner sections cool faster than the rest of the forging.Dead Zones
Improperly formed sections and dead zones can be a result of too little metal in the work piece or flawed forging die design resulting in incorrect material distribution during the process.In general, defects in parts manufactured by metal forging can be controlled first by careful consideration of work stock volume, and by good design of both the forging die, (mold), and the process. The main principle is to enact the right material distributions, and the right material flow to accomplish these distributions. Die cavity geometry and corner radius play a large roll in the action of the metal. Forging die design, and forging process design will be discussed in later sections.
Go back to Forging